Joanna Bloch-Orłowska , Katarzyna Żółkoś , Elżbieta
Transkrypt
Joanna Bloch-Orłowska , Katarzyna Żółkoś , Elżbieta
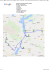
Recovery plan for Saxifraga hirculus in northern Poland – limitations and first results 1 1,2 Joanna Bloch-Orłowska , Katarzyna Żółkoś , Elżbieta Cieślak 3 1 University of Gdańsk, Department of Plant Taxonomy and Nature Conservation, Wita Stwosza 59, 80-308 Gdańsk, Poland 2 University of Gdańsk, Biological Station, Ornitologów 26, 80-680 Gdańsk, Poland 3 W. Szafer Institute of Botany, Polish Academy of Science, Laboratory of Molecular Analyzes, Lubicz 46, 31-512 Kraków, Poland A INTRODUCTION The presented research was the first step of the action, aimed on strengthening existing populations and reintroduction of Saxifraga hirculus in northern Poland. It is a part of AlkFens project, i.e. 'Conservation and restoration of alkaline fens (code 7230) in the young-glacial landscape of the northern Poland', financed from LIFE+ funds and the National Fund for Environmental Protection and Water Management, managed by the Naturalists' Club in cooperation with the Regional Directorates for Environmental Protection in Gdańsk and Olsztyn, Poland. AIM The main aim of the first phase was to recognize actual resources and genetic diversity of chosen populations of Saxifraga hirculus in northern Poland. STUDY SPECIES SAXIFRAGA HIRCULUS - a small herbaceous perennial of circumpolar distribution (Fig. 1, 2), a glacial relic in Poland. The plant is very rare and endangered in Central and Western Europe and threatened in Eastern Europe, although it is a DD species according to European red list. In Poland is endangered and strictly protected by law. It is mostly threatened by expansion of tall perennials and shrubs, usually as a consequence of changes in the water level (drainage) and cessation of extensive mowing. Yellow Marsh Saxifrage usually occurs in alkaline fens, mostly in plant communities of the Scheuchzerio-Caricetea nigrae class or in wet depressions within large sedge aggregations, e.g. Caricetum paniculatae. In Poland the species was reported from over 240 sites, out of which only 28 have been confirmed or discovered during the last twenty years. Most of actual populations are located in N Poland, especially in its north-eastern part (Fig. 3). Size of local populations is highly differentiated from few flowering plants or several dozen to few hundreds or even thousands of individuals. B C D Fig. 1. Saxifraga hirculus: A - flowers, B - flowering stems, C - capsule, D - vegetative stems on the moss and liverworts carpet OR ZS MS MD MR BS MS JK BP PO SM TK STUDY SITES Twelve localities (8 actual and 4 historical) in N Poland (Fig. 3): • in Pomerania: ‘Bagno Stawek' reserve (BS), 'Mechowisko Radość' reserve (MR), Jezioro Krąg (JK), Jezioro Małe Długie (MD), 'Dolina Kulawy' reserve (DK), 'Mechowiska Sulęczyńskie' reserve (MS) and Orle (OR), • in the Chełmno-Dobrzyń Lakeland: Torfowisko Kopaniarze (TK) • in the Suwałki Lakeland: 'Struga Żytkiejmska' reserve (ZS), Poszeszupie (PO), Sawonia-Mostek (SM) and Bagno Parchacz (BP). Fig. 2. Distribution of Saxifraga hirculus in Europe (after Bloch-Orłowska et al. 2014) -1 -2 Fig. 3. Distribution of Saxifraga hirculus in Poland (after BlochOrłowska et al. 2014, modified) 1 - actual population chosen for augmentation, 2 - historical population selected to reintroduction MATERIAL & METHODS Field study was carried out in 2013 and 2014. At each locality the precise position was established (GPS) and permanently marked. Population size was expressed by the area covered by the species and number of flowering stems. For genetic studies fresh, well developed stem leaves were collected. Total DNA of all (161) samples was isolated with using Plant DNeasy Kit (Qiagen, Valencia, CA, USA) according to the manufacturer's instructions. DNA quality and concentration were estimated against λ-DNA on 1% agarose gel stained with ethidium bromide. In AFLP analyses all samples was represented. AFLP generally followed the procedure of Vos et al. (1995), as modified by Cieślak et al. (2007). The three primer combinations for selective PCR were: EcoAAG-MseCTG, EcoACT-MseCAG oraz EcoAGG-MseCAA. In DNA sequencing, each population was represented by 4 to 5 individuals. The three regions of chloroplast DNA (cpDNA) were selected following Oliver et al. (2006). For both PCR and cycle sequencing rbcL, trnD-trnT and trnL-trnF cpDNA primers were used. Products of AFLP and DNA sequencing were separated on an ABI PRISM 3100-Avant automated capillary sequencer, using POP-7 in a 36 cm capillary. Fig. 4. Patch with easy to overlook fruiting stems of Saxifraga hirculus LIMITATIONS OCCURRED DURING THE PROJECT • As a small species Saxifraga hirculus is easy to overlooked among other, taller plants, especially beyond flowering period (Fig. 4). Even few visitations during one growing season are needed to achieve the optimum time for location and precise establishing of population size. • In unfavourable conditions plant reduces the number of inflorescences or doesn't flower at all, which makes it impossible to find. • Yellow Marsh Saxifrage is very fragile to changes between growing seasons. In consequence population size (expressed by number of inflorescences) may fluctuate dramatically or even may be not confirmed. FIRST RESULTS Actual resources of the species Marsh Saxifrage was confirmed within the all 8 actual sites, although in the first year of field studies it was found only at 2 localities and the population sizes noted in 2014 in most cases were much lower than few years ago (Fig. 5). At each locality species occurred in few small, usually closely distributed patches. 120 2008-2010 A Genetic diversity Analysis of main coordinates (PCoA), based on AFLP markers and Nei & Li genetic distance between individuals of Saxifraga hirculus showed diversity among studied populations. The biggest dissimilarity was observed in 3 Pomeranian populations Bagno Stawek (BS), Jezioro Krąg (JK) and Mechowisko Radość (MR). Other populations formed rather dense group, with individuals from particular populations slightly overlapping (Fig. 6). 1 3.9 100 2014 3.1 2.4 80 1.6 SHI_ZS_9 60 0.8 AXIS 2 number of flowering stems 20132 40 -3.9 -3.1 -2.4 -1.6 -0.8 0.8 1.6 2.4 3.1 B BS 19 BS 25 BS In Bayesian analysis in STRUCTURE based on the mean L (K) and K values indicated K = 6 as the most appropriate clustering for the whole data set. For this K value, individuals from 6 populations formed separate and homogeneus genetetic pools (Fig. 7A). Analysis of molecular variance (AMOVA) of Saxifraga hirculis at the level of populations showed that the interpopulation variation made greater contribution than intrapopulation: 55.7% and 44.2% respectively (FST=0.56, P < 0.001). 3.9 64 JK 21 BS 01 JK 25 JK 01 JK JK 06 MD 02 JK 13 MD 03 MD TK 04 TK 07 ZS 18 ZS 10 MR ZS 02 39 TK 22 41 MR 12 PO MR 04 MR 25 SM TK 12 TK 16 PO 01 PO 05 TK PO 13 PO 17 39 * 0 BS JK MD MR TK PO SM ZS population Fig. 5. Changes in population sizes of Saxifraga hirculus during last years (1- unpublished data from the Naturalists’ Club, 2 - P. Pawlikowski’s unpublished data, * - data deficient) -2.4 -3.1 -3.9 AXIS 1 Fig. 6. PCoA ordination graph for the first 2 axes, based on AFLP markers and Nei & Li genetic distance among 161 individuals of Saxifraga hirculus CONCLUSIONS • Due to evanescent occurrence of Saxifraga hirculus in different years, populations should be thoroughly monitored in the optimum of the flowering period. • No strict correlation between genetic and geographic distance were found, however due to the diversity observed among populations in all protection actions connected with strengthening of populations, individuals from particular site should augment the output populations and the reintroductions should be made with the use of material from nearest locality. • Transfer of individuals among sites and regions is inadvisable. PO 22 SM 01 Chełmno-Dobrzyń Lakeland ZS Pomerania 6 -1.6 MR 07 53 Suwałki Lakeland - BS - JK - MD - MR - PO - SM - TK - ZS BS 06 MD 05 -0.8 20 BS 14 SM 03 SM 05 SM 08 SM 09 ZS 01 0.00005 Fig. 7. Genetic diversity of Yellow Marsh Saxifrage populations A - Results of Bayesian analysis in STRUCTURE based on the mean L (K) and K values for K=6 B - The NJ dendrogram of relationship among populations based on Nei's unbiased genetic distance Haplotype variation based on sequencing analysis cpDNA fragment The obtained and analysed sequences covered 2496 bp of three cpDNA fragments (atpB-rbcL- 781 bp, ; trnL(UAA)-trnF(GAA) - 799 bp, trnD-trnT- 916 bp psbA and trnH, 555 bp;, 822 bp; ycf6-psbM, 688 bp). In cpDNA, mutations were recorded, including 7 substitutions and deletions/insertions (e.g. deletions of one nucleotide, deletions of several nucleotides). The NJ dendrogram displayed defined clusters but the bootstrap support was very low (39), confirming lack of strong variation in the data set (Fig. 7B). References: Bloch-Orłowska J., Pawlikowski P., Cieślak E. 2014. Saxifraga hirculus L. Skalnica torfowiskowa. In: Kaźmierczakowa R., Zarzycki K., Mirek Z. (eds.), Polska Czerwona Księga Roślin: paprotniki i rośliny kwiatowe. Polish Red Book of Plants. 3rd ed. IOP Pan, Kraków, p. 246-248. Cieślak E., Ronikier M., Koch M. A. 2007. Western Ukrainian Cochlearia (Brassicaceae) – the identity of an isolated edge population. Taxon 56(1): 112–118. Oliver C., Hollingsworth P.M., Gornall R.J. 2006. Chloroplast DNA phylogeography of the arctic-montane species Saxifraga hirculus (Saxifragaceae). Heredity 96, 222231. doi:10.1038/sj.hdy.6800785 Vos, P., Hogers, R., Bleeker, R., Reijans, M., van de Lee, T., Hornes, M., Frijters, A., Pot, J., Peleman, J., Kuiper, M. & Zabeau, M. 1995. AFLP: a new technique for DNA fingerprinting. Nucl. Acids Res. 23: 44074414. alkfens.kp.org.pl